

Pharmacist compounding and preparing medication on the High-Efficiency Compounding Hood
Practicing safe pharmaceutical compounding helps protect the pharmacist from exposure to harmful substances and particulate, as well as preserve the safety of the medication for the patient. Compounding entails the creation of a drug customized to meet the unique needs of an individual patient (Ref. 1). A compounding pharmacist uses a licensed physician’s prescription to provide patient-specific customized medication such as adjusting the strength or dosage, adding a flavor, reformulating to exclude an ingredient due to allergies, or changing the form of the medicine (Ref. 1).
Compounded drugs have more quality risks than drugs manufactured by FDA-approved manufacturers. To prevent quality issues in compounded medications, licensed pharmacists must implement safety guidelines to understand potential pitfalls, improve the quality of the final product, and reduce patient health risks (Ref. 2). Likewise, proper compounding procedures can help protect the pharmacist and other staff from exposure to harmful substances and particulate.
Health Risks of Pharmaceutical Compounding
Health risks of pharmaceutical compounding include threats to patient safety as well as the compounding pharmacists. Compounding medication is not regulated with the same scrutiny by the FDA as commercially available medication and can cause health risks to the patient depending on (Ref. 2):
1. Quality of the medication (proper identification, purity, and strength)
2. Environment conditions (using proper compounding areas with proper airflow, sanitation, reducing airborne particulate, and hygiene)
3. Personnel activities (human mistakes, following protocols, and expiration dates)
4. Control process (monitoring the process)
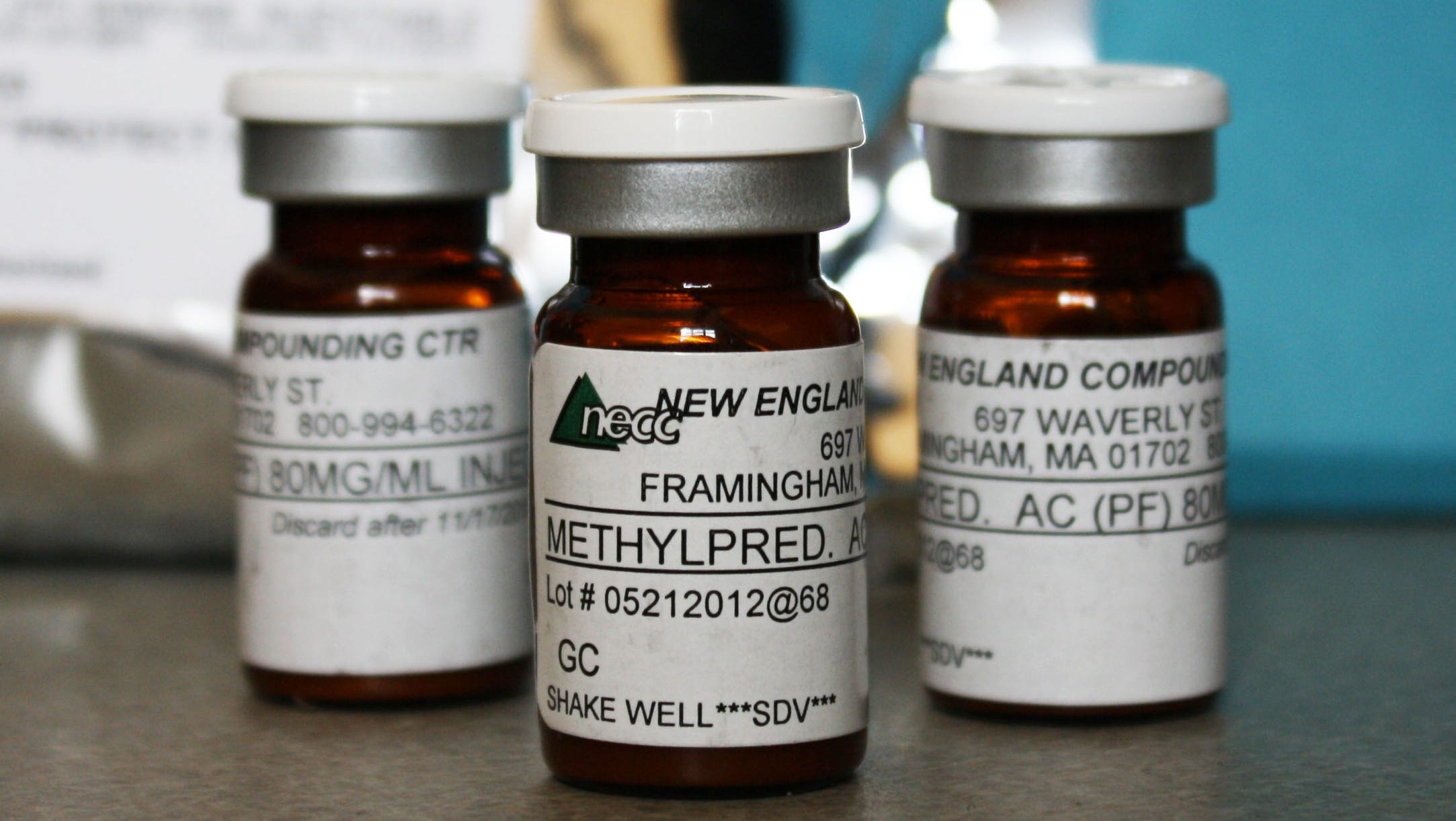
In 2012, contaminated spinal injections from New England Compounding Center caused a massive meningitis outbreak (Source)
For example, unsanitary conditions such as vermin, visible bacteria or microbial contamination, and foreign matter during compounding can lead to patient infections or death (Ref. 3). Notably, in 2012 contaminated spinal injections prepared by New England Compounding Center caused a meningitis outbreak that resulted in 64 deaths and about 753 total affected patients (Ref. 4). The compounding pharmacy broke several regulations by distributing large quantities across state borders, creating a suspension that could not be filtered for bacteria or fungi, and several unsanitary conditions present at the facilities (Ref. 4).
Also, without proper compounding containment enclosures, compounding pharmacists may be at risk for developing health conditions from exposure to harmful drug particulate. While compounding, certain activities generate particulate that can easily be inhaled by the pharmacists leading to possible side effects especially when dealing with hazardous drug (HD) substances. Exposure to HD’s while compounding can lead to skin rashes, infertility, miscarriages, and even cancer in healthcare workers (Ref. 5).
Pharmacy Compounding Regulations
Pharmaceutical compounding facilities do not follow the same regulations as drug manufacturers. Compounded medications are not FDA approved and can’t be verified for efficacy or safety (Ref. 2). Individual state boards of pharmacy mainly regulate compounding, but as a result of the massive meningitis outbreak, the Federal Drug Administration (FDA) created the Drug Quality and Security Act (DQSA) of 2013 to address concerns of compounded drug safety (Ref. 1). This act added specific compounding safety practices, exporting guidelines, and established the FDA as an authority over compounding practices (Ref. 1). The DQSA limits facilities manufacturing and distribution quantity for all compounded drugs to preserve quality and safety (Ref. 1).
The DQSA added sections 503A and 503B to the Federal Food, Drug, and Cosmetic Act of 1938 but allowed certain exemptions to the act as long as the compounding pharmacy follows the new applicable section. Please see the chart below for a comparison of the specific regulations.
| Topic | 503A | 503B |
| Applicable Type of Compounder | Compounding by licensed pharmacists within a state-licensed pharmacy | Outsourcing facilities by licensed pharmacists not required to be a licensed pharmacy |
| Exemptions | 501 – Current Good Manufacturing Practice Requirements 502 – Labeling with Adequate Directions for Use 505 – New Drug Approval Requirements |
502 – Labeling with Adequate Directions for Use 505 – New Drug Approval Requirements 582 – Drug Supply Chain Security Requirements |
| Prescription | Based on prescription for an individual patient, limited quantity, and occurs after receipt of a prescription | May, or may not have prescription |
| Requirements | Not on FDA withdrawn list Not on the list that is known to present difficulties for compounding Not a copy of a commercially available drug |
|
| Interstate Distribution | Limited to 5% or more depending on state guidelines | No regulations |
| Wholesale | No conditions outlined | Cannot be sold or transferred to another entity |
| Registration | No conditions outlined | Compounded in a facility that meets registration and reporting requirements |
| Adverse Effects | No conditions outlined | Must report adverse effects to FDA |
| Labeling | No conditions outlined | Must contain certain information including “This is a compounded drug” on the label |
The United States Pharmacopeia (USP) creates and updates compounding standards for the identity, quality, strength, purity, supplements, and food ingredients used in compounding medications (Ref. 1). The most relevant regulations include:
• USP 797 – sterile preparations – to prevent microbial contamination
• USP 795 – nonsterile preparation – including beyond use date and stability
• USP 800 – hazardous drug compounding – to minimize exposure
USP 795
USP 795 specifies compounding practices for nonsterile preparations. This regulation does not explicitly specify the facility and enclosure requirements but describes the minimum specifications as (Ref. 4):
• Designate a separate and distinct compounding area for nonsterile compounding removed from the sterile compounding area.
• Maintain a clean, orderly, and sanitary compounding area with all equipment in a good state of repair.
• Utilize heating, ventilation, and air conditioning systems properly to avoid decomposition and containment of chemicals.
USP 800
USP 800 outlines best practices for working with hazardous drugs in order to minimize exposure, improve worker and patient safety, and promote environmental protection (Ref. 1). USP 800 specifies how healthcare personnel should handle, compound, administer, store, and transfer HD drugs. For compounding, all particle generating activities must be completed in specific engineering controls to prevent exposure. For primary engineering controls, USP 800 requires an externally vented enclosure of a redundant HEPA filtered hood (Ref. 6). For secondary engineering controls, the external room must be externally vented with 12 air changes per hour and a negative pressure between 0.01 and 0.03 wic (inches of water column) (Ref. 6).
Modular Pharmacy Hoods for Pharmaceutical Compounding

Pharmacist safely compounding hazardous drugs with the High-Efficiency Compounding Hood
Modular pharmacy hoods contain particle generating compounding by using a powerful fan to draw harmful particulates and powders out of the pharmacist’s breathing zone into the high-quality filtration chamber. The high-quality filters remove up to 99.97% of particles down to 0.3 microns for HEPA filters and up to 99.9995% of particles down to 0.12 microns before releasing the filtered air into the surrounding room. Modular pharmacy hoods help protect the operator from exposure and reduce the chance of developing health effects from inhalation. These easy-to-use systems offer environmental protection, energy efficiency, and minimal required maintenance. Please note: Sentry Air Systems hoods are not for sterile compounding.
Pharmaceutical Compounding Hood
High-Efficiency Compounding Hood
Contact us today to learn how Sentry Air can help you compound safely!
Call us today at 1-800-799-4609
Related Links
Powder Containment Hoods | What is USP 800?
Sources
1. “Frequently Asked Questions about Pharmaceutical Compounding.” American Pharmacists Association. 2021. https://www.pharmacist.com/Practice/Patient-Care-Services/Compounding/Compounding-FAQs.
2. Demetzos, Costas; Pippa, Natassa; Siamidi, Angeliki; “Pharmaceutical compounding: Recent advances, lessons learned and future perspectives.” Global Drugs and Therapeutics. 2017. https://www.oatext.com/Pharmaceutical-compounding-Recent-advances-lessons-learned-and-future-perspectives.php.
3. “Insanitary Conditions at Compounding Facilities.” U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. 2020. https://www.fda.gov/media/124948/download.
4. Kienie, Patricia C. “Compounding nonsterile preparations: USP 795 and 800.” Pharmacy Today, October 2017. https://www.pharmacytoday.org/action/showPdf?pii=S1042-0991%2817%2931481-0.
5. “Hazardous Drug Handling in the Community Pharmacy.” Pharm Compliance, 2019. https://www.pharmcompliance.com/post/hazardous-drug-handling-in-the-community-pharmacy.
6. “USP 800.” United States Pharmacopeia, 2019. https://www.usp.org/compounding/general-chapter-hazardous-drugs-handling-healthcare.

 Made in the USA
Made in the USA







