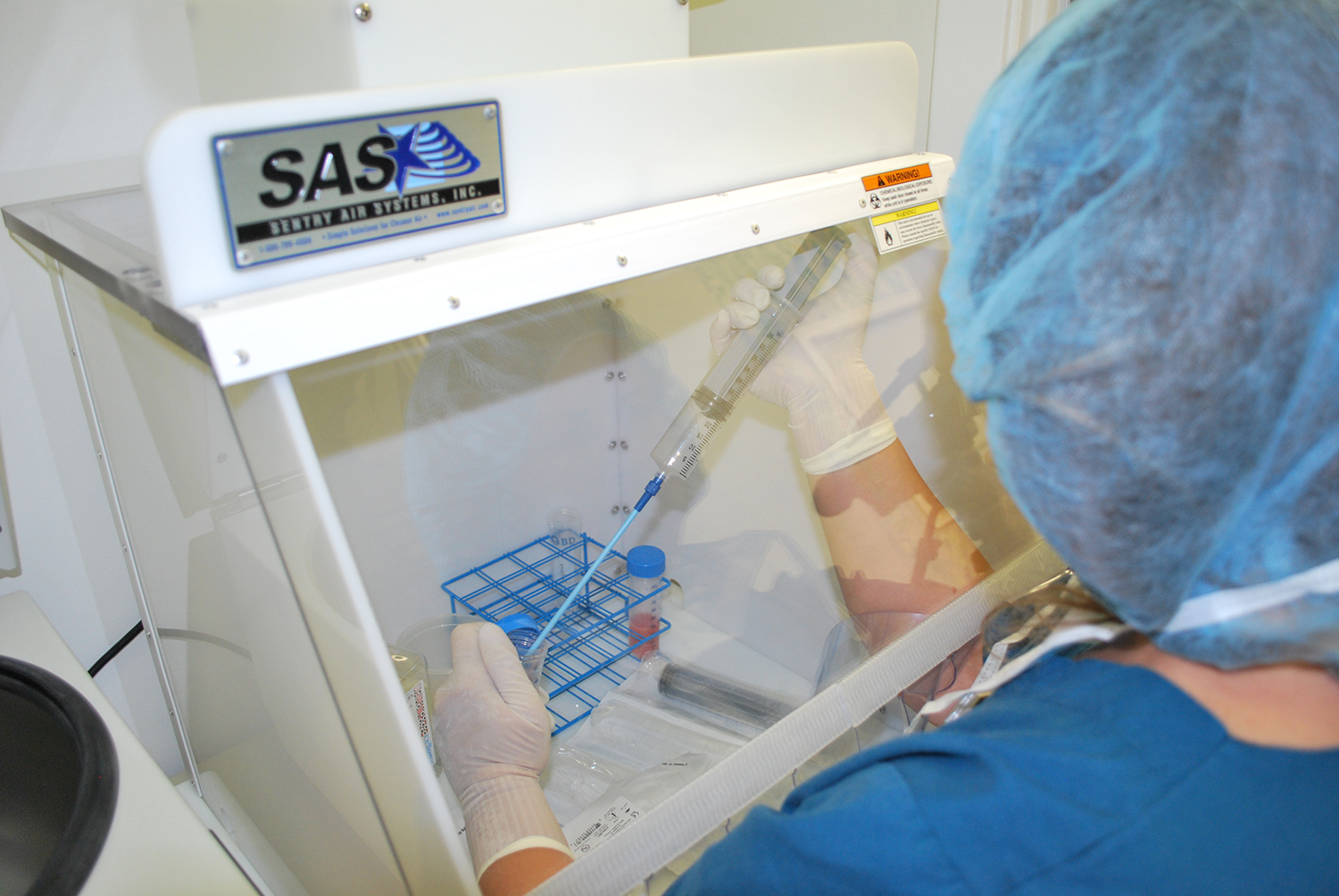
Laminar Flow Hoods help protect PCR samples from outside contamination.
Polymerase Chain Reaction (PCR) requires strict laboratory protocol and procedures to prevent contamination and ensure accurate results (Rhea-McManus, 2022). PCR has revolutionized molecular biology and gene studies but recently became widely known for pathogen tests such as COVID-19 (Rhea-McManus, 2022). Laboratories should utilize Laminar Flow Hoods or Portable Clean Rooms for preparation and other sensitive PCR steps in order to help protect samples from outside contamination (WHO, 2018).
What is Polymerase Chain Reaction?
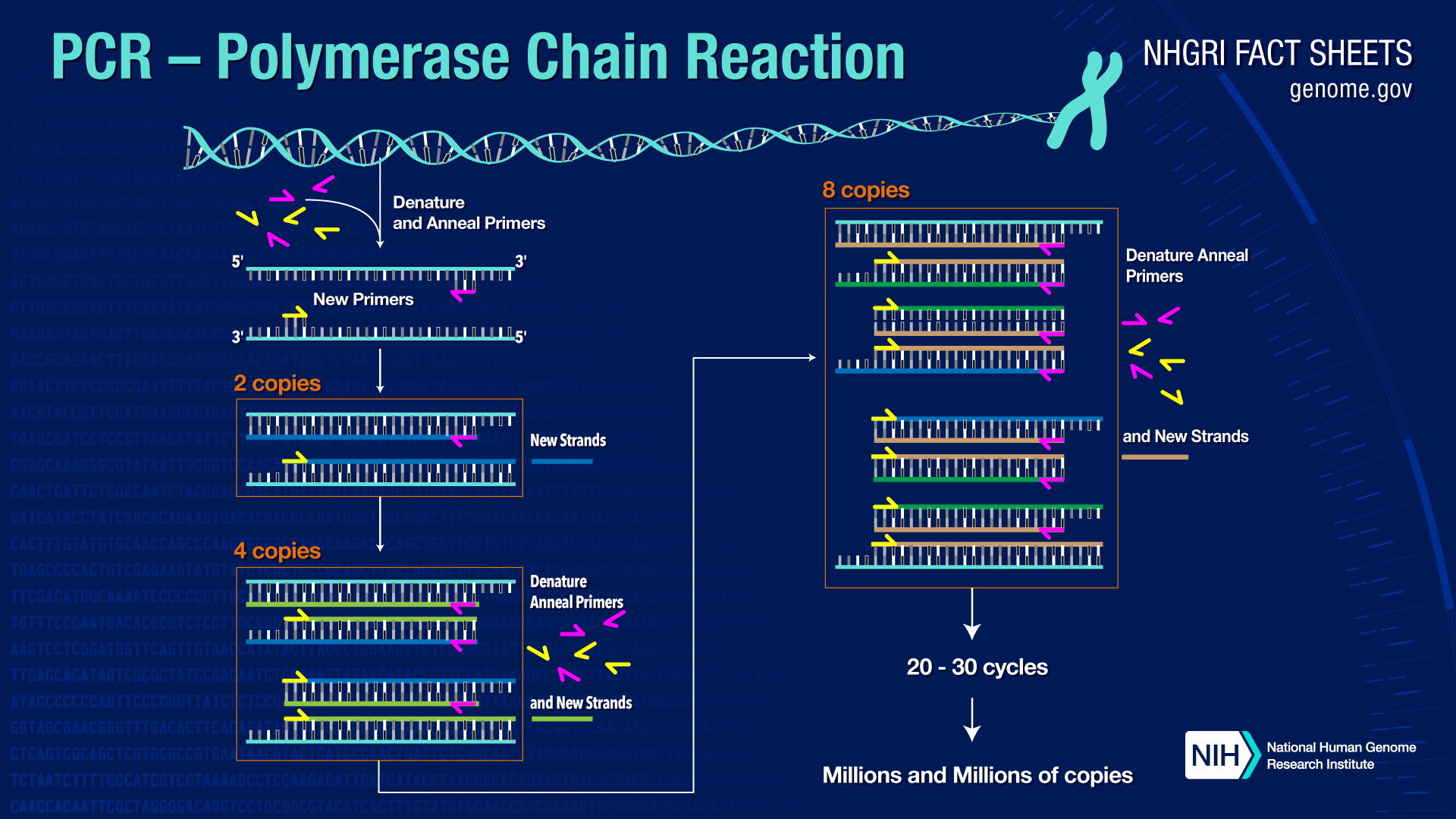
Illustration of the PCR Process from Human Genome Project (Source)
Polymerase Chain Reaction, or PCR, is a common laboratory procedure that amplifies, or copies, a specific sequence of DNA (Rhea-McManus, 2022). PCR was developed in 1983 by Kary Mullis (Bio-Rad). PCR allows researchers to select a specific sequence of DNA to study and can even detect single molecules of DNA (Rhea-McManus, 2022). The sensitivity of this procedure makes it highly susceptible to contamination (Rhea-McManus, 2022). PCR is an automated process consisting of a 3-step reaction:
1. Template DNA, or the specific sequence of DNA that researchers want to study, is heated to cause the DNA strands to denature or separate into single strands (Bio-Rad).
2. The heat is reduced allowing the template DNA to bind to the primers (Bio-Rad).
3. Heat is increased allowing the heat-stable polymerase (enzyme) to synthesize and build 2 new strands of DNA (NCBI).
This 3-step reaction repeats 30-40 times and results in exponential growth with billions of amplicons, or copies, of the template DNA (NIH, 2020).
Applications of PCR
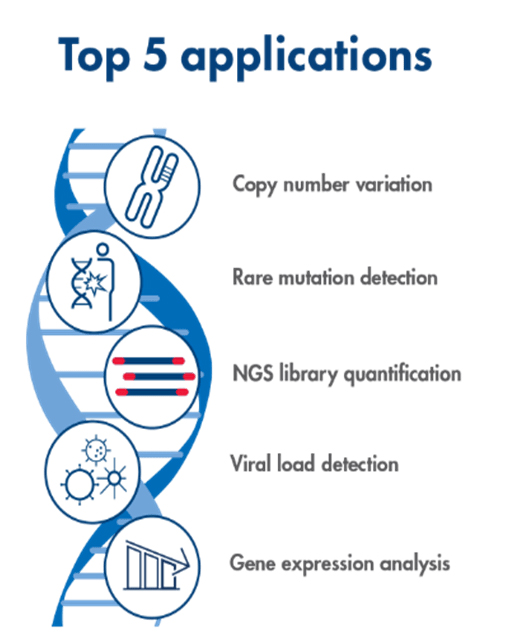
Top 5 PCR applications from University of Texas in Arlington (Source)
PCR allows researchers to study small segments of DNA ideal for numerous medical, forensic, and agricultural applications (ThermoFischer). Below are some of the many common applications for PCR:
Genetic Testing (ThermoFischer)
Genotyping (ThermoFischer)
Cloning (ThermoFischer)
Analyzing archaeological specimens (Lenstra, 1995)
Mapping hereditary traits (Lenstra, 1995)
Finding drug resistant mutations in viruses, bacteria, and fungi (Aslanzadeh, 2004)
Mutation Testing (Bio-Rad)
Paternity Testing (Bio-Rad)
Pathogen Testing (such as COVID-19, HIV, Lyme disease) (Munawar, 2022)
Genetic Disorder Testing (NIH, 2020)
DNA Fingerprinting (NIH, 2020)
Food Pathogen Detection (ThermoFischer)
Plant Genotyping (ThermoFischer)
Where does PCR contamination occur?

Lab Technician using a Laminar Flow Hood to prepare PCR samples (Source)
Contamination in PCR can occur during amplification set-up, amplification product handling, aerosol DNA, and storage (Scherczinger, 1999). The main types of contamination are between samples, between nucleic acids, and through amplicon carryover (Rhea-McManus, 2022). Cross-contamination between samples can occur when handling samples before the PCR process. Contamination sources can be from reagents, disposable supplies, and improper handling procedures (Hu, 2016). Also, nucleic acids can cross-contaminate from past samples and from organisms that are present in the lab (Hu, 2016). Lastly, the most important contamination source is from amplification carryover. Amplicons can contaminate laboratory equipment, surfaces, and even the lab’s ventilation system (Hu, 2016).
Importance of Minimizing PCR Contamination
It is very important for labs to minimize and prevent contamination during and after the PCR process to provide consistent and accurate results (Rhea-McManus, 2022). In the medical field, PCR contamination can affect patient care with false positive results (Das, 2018). False disease reports can lead to unnecessary medical treatment. In clinical history, there have been two patients misdiagnosed with Lyme disease due to a false positive PCR test (Aslanzadeh, 2004). Both received treatment and one patient passed away as a result (Aslanzadeh, 2004). Contamination can also waste valuable lab resources when identifying the contamination source and thoroughly cleaning the lab to remove contamination sources (Rhea-McManus, 2022).
Methods to Minimize PCR Contamination
Utilizing careful lab techniques and good quality control practices can help reduce most PCR contamination (Rhea-McManus, 2022). The World Health Organization and other lab professionals recommend the following practices to help prevent PCR contamination (WHO, 2018).
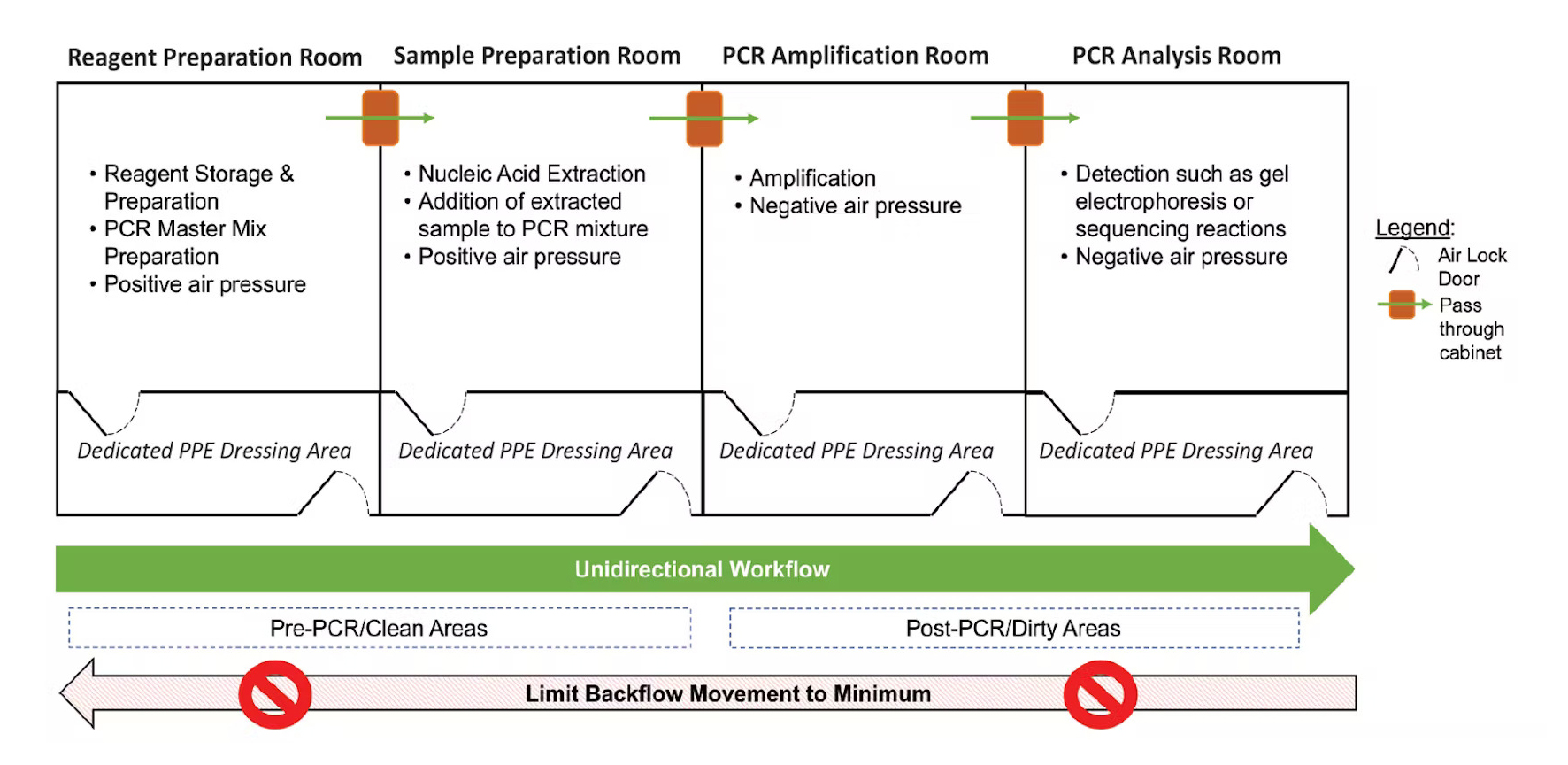
Ideal Lab Set-up to Reduce PCR Contamination (Source)
Lab Design
Using separate areas for preparation and post-PCR activities can significantly reduce carryover contamination (WHO, 2018). Ideally, the lab would have 3-4 different rooms, however, two separate rooms or workbenches for pre and post-PCR would be enough to help reduce contamination (Rhea-McManus, 2022).
Unidirectional Workflow
Lab technicians should only move from clean rooms to dirty rooms, or pre-PCR to post-PCR rooms, to reduce contamination risks. If you must go backward, proper lab hygiene such as washing hands, changing lab coats, and not moving dedicated lab equipment can help mitigate risks (Rhea-McManus, 2022).
Dedicated Lab Supplies for each Room/Area
Labs should use dedicated lab supplies for each room including lab coats, pipettes, workbenches, tube racks, gloves, lab reagents, etc. (WHO, 2018).
Cleaning and Sanitizing Procedures
All lab surfaces, equipment, and hoods should be cleaned after use for PCR procedures. Cleaning techniques include using chemical cleaners, UV light, and enzymes (Hu, 2016). UV light and enzymes deactivate DNA to prevent carryover contamination.
Laminar Flow Hoods
Laminar Flow Hoods should be used while preparing samples and analyzing post-PCR products (Rhea-McManus, 2022). These systems filter out external contamination by supplying filtered air to the workspace. The next section will go into more detail about how Laminar Flow Hoods can help prevent PCR contamination.
Using Laminar Flow Hoods to prevent PCR Contamination

Safely prepare samples for PCR and other sensitive steps by using a Laminar Flow Hood, shown here Standard Portable Clean Room from Sentry Air.
Laminar Flow Hoods (also known as PCR workstations or Portable Clean Rooms) provide a reduced particulate workspace for sensitive applications such as polymerase chain reaction. These systems draw ambient air into the filtration system and create an ISO Class 5 Cleanroom. For preventing PCR contamination, Laminar Flow Hoods are recommended for the following parts of the PCR process:
• Pre-PCR to mix reagents and prepare samples (WHO, 2018)
• Adding DNA to PCR reaction mixes (Rhea-McManus, 2022)
• For nested PCR reactions when adding round 1 PCR to round 2 reaction (WHO, 2018)
• Opening amplicon tubes after PCR (Rhea-McManus, 2022)
Sentry Air Portable Clean Rooms
Portable Clean Rooms can help protect PCR samples from outside contamination by creating a sterile workspace. Portable Clean Rooms offer dual-stage filtration with a pre-filter and main filter. Main filter choices include HEPA (up to 99.97% efficiency on particles 0.3 microns and larger) or ULPA (up to 99.9995% efficiency on particles 0.12 microns and larger). Also, these systems can come with a UV light to decontaminate the worksurface. The chart below summarizes the features and benefits of our standard Portable Clean Room line and our UVC Portable Clean Room line.
 |
 |
|
| Product Series Name | Standard Portable Clean Rooms | UVC Portable Clean Rooms |
| Benefits | ISO Class 5 Cleanroom Dual-stage Filtration Positive-Pressure Environment Vinyl Curtains to provide easy access while keeping air inside |
|
| UVC Light | Optional | Standard with digital control panel featuring adjustable airflow and LED light, runtime, and 15-minute UVC cycle with alarm and countdown for operator safety. |
| Filters | Pre-filter: MERV 8 Pre-Filter Main Filters: HEPA (up to [up to 99.97% efficiency on particles 0.3 microns and larger) or ULPA (up to 99.9995% efficiency on particles 0.12 microns and larger) |
|
| Sizes Available | 12”, 18”, 24”, 30”, and 40” | 24”, 30”, and 40” |
| Airflow | 12” – up to 100 CFM 18 – up to 65 CFM 24”, 30”, and 40” – up to 350 CFM |
Up to 350 CFM |
Please note: This information is provided as a customer service. All information provided should be independently verified.
Help Prevent PCR Contamination with Portable Clean Rooms from Sentry Air! Contact us today to get started.
Call today – 1.800.799.4609
Related Blogs
• Rotary Evaporator Fume Control
• Mycology Culture Sampling & Testing
• Stool Culture + Sentry Air Systems
• Laboratory Fume Hoods
References
Aslanzadeh, J. (2004, July 28). Preventing PCR Amplification Carryover Contamination in a Clinical Laboratory. Annals of Clinical & Laboratory Science. Retrieved from: http://www.annclinlabsci.org/content/34/4/389.long.
Bio-Rad. PCR (Polymerase Chain Reaction). Retrieved from: https://www.bio-rad.com/en-us/applications-technologies/pcr-polymerase-chain-reaction?ID=LUSNYI15.
Das, P. & Ganguly, S. & Mandal, B. (2018, December 18). Mitigating polymerase chain reaction/amplicon contamination in a high-risk high-burden mycobacterial reference laboratory in a resource-limited setting. International Journal of Mycobacteriology. Retrieved from: https://www.ijmyco.org/text.asp?2018/7/4/332/246923.
Hu, Y. (2016, October 12). Regulatory Concern of Polymerase Chain Reaction (PCR) Carryover Contamination. Polymerase Chain Reaction for Biomedical Applications. doi: 10.5772/66294. Retrieved from: https://www.intechopen.com/chapters/52932
Lenstra, J. (1995, July). The applications of the polymerase chain reaction in the life sciences. PubMed. PMID: 7580841. Retrieved from: https://pubmed.ncbi.nlm.nih.gov/7580841/.
Munawar, M. (2022, March 16). Smart PCR machines can reduce the risk of carryover contamination. BioTechniques<, Vol. 72, No. 3. Retrieved from: https://www.future-science.com/doi/10.2144/btn-2021-0064.
NCBI. Polymerase Chain Reaction (PCR). National Center for Biotechnology Information. Retrieved from: https://www.ncbi.nlm.nih.gov/probe/docs/techpcr/.
NIH. (2020, August 17). Polymerase Chain Reaction (PCR) Fact Sheet. National Human Genome Research Institute. Retrieved from: https://www.genome.gov/about-genomics/fact-sheets/Polymerase-Chain-Reaction-Fact-Sheet.
Rhea-McManus, J. (2022, April 20). Preventing Cross-contamination in a Molecular Laboratory. Medical Laboratory Observer. Retrieved from: https://www.mlo-online.com/management/qa-qc/article/21264285/preventing-crosscontamination-in-a-molecular-laboratory.
Scherczinger, C. A., Ladd, C., Bourke, M. T., Adamowicz, M. S., Johannes, P. M., Scherczinger, R., Beesley, T., & Lee, H. C. (1999, September). A systematic analysis of PCR contamination. Journal of Forensic Sciences, 44(5), 1042–1045. Retrieved from: https://pubmed.ncbi.nlm.nih.gov/10486955/.
ThermoFischer. PCR Applications–Top Seven Categories. Retrieved from: https://www.thermofisher.com/us/en/home/life-science/cloning/cloning-learning-center/invitrogen-school-of-molecular-biology/pcr-education/pcr-reagents-enzymes/pcr-applications.html.
World Health Organization. (2018, January 31). Dos and Don’ts for molecular testing. Global Malaria Programme. Retrieved from: https://www.who.int/teams/global-malaria-programme/case-management/diagnosis/nucleic-acid-amplification-based-diagnostics/dos-and-don-ts-for-molecular-testing.

 Made in the USA
Made in the USA



