What is Hydrochloric Acid?
Hydrochloric acid is a colorless, corrosive, liquid that fumes in air at high concentrations of 25% or more, and becomes a hydrogen chloride gas forming dense white vapors due to condensation with atmospheric moisture (Pubchem). The vapor is corrosive, and air concentrations above 5 ppm can cause irritation. Hydrogen chloride is available commercially as an anhydrous gas or as aqueous solutions (hydrochloric acid). Synonyms for an aqueous solution of hydrogen chloride include chlorhydric acid, hydrochloric acid, and muriatic acid.
What Does Hydrochloric Acid React With?
As an aqueous solution, HCl reacts exothermically with organic bases (amines, amides) and inorganic bases (oxides and hydroxides of metals). Reacts exothermically with carbonates (including limestone and building materials containing limestone) and hydrogen carbonates to generate carbon dioxide. Reacts with sulfides, carbides, borides, and phosphides to generate toxic or flammable gases. Reacts with many metals (including aluminum, zinc, calcium, magnesium, iron, tin and all of the alkali metals) to generate flammable hydrogen gas. Reacts violently with acetic anhydride, 2-aminoethanol, ammonium hydroxide, calcium phosphide, chlorosulfonic acid, 1,1-difluoroethylene, ethylenediamine, ethyleneimine, oleum, perchloric acid, b-propiolactone, propylene oxide, silver perchlorate/carbon tetrachloride mixture, sodium hydroxide, uranium(IV) phosphide, vinyl acetate, calcium carbide, rubidium carbide, cesium acetylide, rubidium acetylide, magnesium boride, mercury(II) sulfate (Cameo Chemicals). Mixtures with concentrated sulfuric acid can evolve toxic hydrogen chloride gas at a dangerous rate. Undergoes a very energetic reaction with calcium phosphide (Hydrochloric Acid SDS).
History of Hydrochloric Acid
Hydrochloric acid (HCl) was first discovered around 800 C.E. by the alchemist Jabir ibn Hayyan (Geber), by mixing common salt with vitriol (sulfuric acid) (ReAgent). Jabir discovered many important chemicals, and recorded his findings in over 20 books, which carried his chemical knowledge of hydrochloric acid and other basic chemicals for hundreds of years. Jabir’s invention of the gold-dissolving aqua regia, consisting of hydrochloric acid and nitric acid, was of great interest to alchemists searching for the philosopher’s stone.
Hydrochloric Avid vs. Hydrogen Chloride
Hydrogen chloride is the gaseous form of H-Cl while hydrochloric acid is the aqueous form (H-Cl dissolved in water). Hydrogen chloride is produced commercially by any of the following reactions: heated hydrogen gas with calcium chloride, sulfuric acid with sodium chloride, sodium chloride with sulfur dioxide and steam, and hydrogen burned in chlorine (ATSDR). Hydrogen chloride can be formed during the combustion of many plastics. Hydrochloric acid (muriatic acid) is a component of commercial chemicals used to clean and disinfect swimming pools. Hydrogen chloride is used for cleaning, pickling, and electroplating metals; in refining mineral ores; in petroleum well extraction; in leather tanning; and in the refining of fats, soaps, and edible oils and as a digestate for tissue sampling. It is also used in producing polymers and plastics, rubber, fertilizers, dyes, dyestuffs, and pigments.
Applications of Hydrochloric Acid
Applications of HCl is widely used as a laboratory reagent (34-37% reagent grade). Frequently, the sample is not soluble in water and must be treated with acids or a mixture of acids to facilitate solubility. Tissue samples are digested in HCl and that digestate is examined for the presence of metals.
Hydrochloric Acid in Stomach
Not many people are aware of the naturally occurring form of hydrochloric acid produced by the lining of the stomach, that breaks down connective tissue and cell membranes in the food, so that it can more easily be acted on by digestive enzymes. Hydrochloric acid also kills most of the bacteria ingested with the food. It can be responsible for gastroesophageal reflux disease and just plain ole acid indigestion.
Health Side Effects of Exposure of Hydrochloric Acid
When heated, hydrochloric acid generates large quantities of fumes. If the concentration of hydrochloric acid gas in the air is 0.035%, humans will have a pain in the throat and chest, and have difficulty in breathing within 10 minutes. The inhalation of a large quantity of hydrochloric acid gas or mist may result in death. The chart below summarizes possible side effects for certain concentrations of hydrochloric acid fumes.
| Severity | Symptom | Concentration Level (ppm) |
| Allowable Concentrations | Long-term Sustainable Limit | 5 |
| Mild Symptoms | Production of tears, coughing, sneezing, and runny nose. | 10 – 50 |
| Moderate Symptoms | Difficulty in breathing, difficulty in opening the eyes, chest pain, life threatening in 30 minutes to one hour. | 50 – 100 |
| Serious Symptoms | Impossible to breathe, unconsciousness, death in 30 minutes to one hour | 1,000 – 1,300 |
| Lethal Dose | 1,300 – 2,000 (0.13 – 0.2%) |
Occupational exposure of Hydrochloric Acid
According to the EPA, occupational exposure can rapidly lead to swelling and spasm of the throat and suffocation. Material is extremely destructive to tissue of the mucous membranes and upper respiratory tract. Inhalation of hydrochloric acid vapors and mists produces nose, throat, and laryngeal burning, and irritation, pain and inflammation, coughing, sneezing, choking sensation, shortness of breath, hoarseness, laryngeal spasms, upper respiratory tract edema, bronchial constriction, bronchitis, chest pains, as well has headache, and palpitations. Inhalation of high concentrations can result in corrosive burns, necrosis of bronchial epithelium, constriction of the larynx and bronchi, nasospetal perforation, glottal closure, occur, particularly if exposure is prolonged. May be fatal if inhaled.
Controlled Hydrochloric Acid Exposure Testing on Humans
Human asthmatics (5/sex) were exposed to 0.8 or 1.8 ppm HCl for 45 minutes, and pulmonary function tests performed immediately after exposure were compared to baseline levels (Stevens et al., 1992). No exposure-related effects were observed in subjective symptoms or in pulmonary function tests, including forced expiratory volume in 1 second, forced vital capacity, maximal flow at 50 and 75% of vital capacity, respiratory resistance, and peak flow. This is the only available controlled human exposure study of HCl (Hydrogen chloride).
Testing Sentry Air Systems’ Effectiveness of Filtering Inorganic Acids
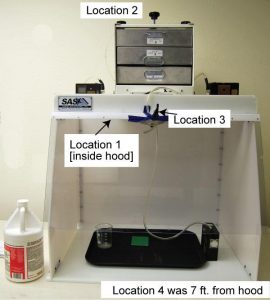 A test was set up using Sentry Air’s 30″ Wide Ductless Fume Hood Model # SS-330-DCH in accordance with NIOSH Test Method 7903 to test for airborne concentrations of inorganic acids, in this case HCl. (This method can be viewed on the NIOSH website: http://www.cdc.gov/niosh/docs/2003-154/pdfs/7903.pdf) The test objective was to determine the effectiveness of Sentry Air Systems’ Acid Gas filter media in keeping operator ambient hydrochloric acid concentrations to a minimum while the interior of the hood was subjected to a large quantity of hydrochloric acid fumes.
A test was set up using Sentry Air’s 30″ Wide Ductless Fume Hood Model # SS-330-DCH in accordance with NIOSH Test Method 7903 to test for airborne concentrations of inorganic acids, in this case HCl. (This method can be viewed on the NIOSH website: http://www.cdc.gov/niosh/docs/2003-154/pdfs/7903.pdf) The test objective was to determine the effectiveness of Sentry Air Systems’ Acid Gas filter media in keeping operator ambient hydrochloric acid concentrations to a minimum while the interior of the hood was subjected to a large quantity of hydrochloric acid fumes.
Note: This method should no longer be used as it classified as a historical document and has been replaced by NIOSH methods 7906, 7907, & 7908. The replacement methods (7906, 7907, & 7908) allow for the collection of inhalable fractions of acid aerosols by means of a prefilter and can provide for lower limits of detection for acid gases and vapors due to higher sampling flow rates.
The results of this test are unequivocal in recommending the Acid Gas filter for use in applications involving the use of, or potential exposure to, hydrochloric acid. The filter removed, as nearly as can be determined, all acid from the airstream being treated and reduced the potential operator exposure from upwards of 50 ppm at Location 1 to a concentration at Location 2 that is less than the detection limits of the analysis methods used (approximately 0.003 ppm). No hydrochloric acid was detected in the ambient room atmosphere throughout the test (Location 4), nor was any detected at the operator’s breathing location (Location 3), nor at the outlet of the hood (Location 2). Taking into account potential error in the sample analysis and the analysis method’s detection limits, the filter appears to have removed >99.99% of the hydrochloric acid fumes.
Results
| Chemical Tested | Sample Location | Amount Absorbed by Sorbent Tube | Volume Sampled | Average Sample Concentration | Concentration |
| HCl Solution | Blank | < 0.006 mg | n/a | n/a | — |
| HCl Solution | Location 1 (Inlet) |
0.74 mg | 7.8 L | 94.4 mg/m3 | 58.6 ppm |
| HCl Solution | Location 3 (Operator Ambient) |
< 0.006 mg | 7.5 L | 0.8 mg/m3 | < 0.003 ppm |
| HCl Solution | Location 2 (Outlet) |
< 0.006 mg | 7.6 L | 0.8 mg/m3 | < 0.003 ppm |
| HCl Solution | Location 4 (Ambient Room) |
< 0.006 mg | 7.6 L | 0.8 mg/m3 | < 0.003 ppm |
Engineering Controls for Hydrochloric Acid

Our ductless containment hoods, like the Model 330 shown here, pulls respiratory hazards into filters away from the operator’s breathing zone.
Sentry Air’s 30″ Wide Ductless Fume Hood Model # SS-330-DCH with 10lb acid gas filter combines powerful air flow with a sturdy and compact enclosure for optimal respiratory and environmental protection. This fume hood is designed to pull harmful fumes and particulate up and away from the operator’s breathing zone and into the filter chamber. For hydrochloric acid applications, Sentry Air recommends using a Carbon Pre-filter and 10lb Acid Gas Filter to adsorb fumes. For other applications, the filter chamber can house either HEPA filtration [up to 99.97% efficient on particles 0.3 microns and larger and made with flame-retardant materials], ASHRAE filtration [up to 95% efficient on particles 0.5 microns and larger and made with flame-retardant materials] Activated Carbon, or specialty-blended filter media [i.e. Acid Gas, Mercury, Aldehyde, Ammonia]. Variable Speed Control and a Fluorescent Light come standard with this unit.
Other typical uses for this fume hood include chemical fume control, pharmaceutical compounding containment, soldering applications, light dust removal, biological applications, solvent or epoxy use, and many more applications that require the removal of fumes and particulate.
Contact Us:
For more information about controlling hazardous fumes, contact Sentry Air and speak with one of our applications specialists.



Call today – 1.800.799.4609
References
ATSDR. Medical Management Guidelines for Hydrogen Chloride. Toxic Substanced Portal. 2014 October 21https://wwwn.cdc.gov/TSP/MMG/MMGDetails.aspx?mmgid=758&toxid=147
Cameo Chemicals. Hydrochloric Acid, Solution Chemical Datasheet. https://cameochemicals.noaa.gov/chemical/3598
EPA. Hydrogen Chloride. Integrated Risk Information System Chemical Assessment. https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0396_summary.pdf#nameddest=rfc
Hydrochloric Acid Safety Data Sheets. EChemi.com. https://www.echemi.com/sds/hydrochloric-acid-pid_Rock19088.html
PubChem. Hydrochloric Acid. National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/Hydrochloric-Acid
ReAgent. What is Hydrochloric Acid? The Chemistry Blog. 2018 June 7.https://pubchem.ncbi.nlm.nih.gov/compound/Hydrochloric-Acid
Related Blogs:
• Laboratory Fume Hoods
• Using Filtration to Help Address Common Exhaust Hood Issues – Exhaust Filter Box
• Testing dairy products requires acid gas filter for fume extraction

 Made in the USA
Made in the USA
